Topics:
-Why
Study Sickle Cell Disease?
-Our Approach
Why Study Sickle Cell Disease?
Sickle cell disease has often been
called a 'molecular disease' because it results from the mutation of one
amino acid. In the Mukerji
lab our goal is to understand this disease on a molecular level. By
studying the structure and energetics of sickle cell hemoglobin fibers,
our research is directed towards understanding the mechanism of sickle
cell hemoglobin fiber formation. By
gaining an understanding of fiber formation on this level, we can begin to
design better and more effective agents to inhibit fiber formation and
disrupt fibers. Fiber formation lies at the root of sickle cell disease;
therefore, understanding this process is inherent to understanding sickle
cell disease.
At the most basic level, we are
interested in understanding the forces that govern the association of
proteins. In particular, we are interested in the self-association of
proteins that leads to the formation of fibrils. Many diseases such as Alzheimer's disease and 'Mad Cow'
disease result from the association or aggregation of proteins into
fibrils. Thus, much of the
basic information gained from this research will be relevant towards
understanding the assembly of proteins into larger structures, functional
and non-functional. Furthermore,
the methodologies we develop to study sickle cell hemoglobin may be
applied to the study of similar problems.
| Deoxy Hb S fibers | Amyloid fibers | Prion fibers |
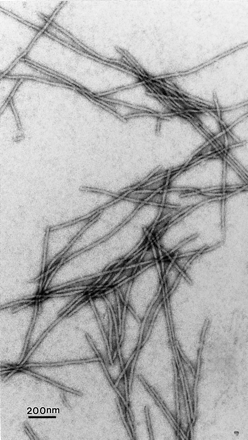 Fig 6.1 |
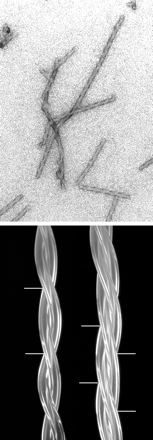 Fig. 6.3 |
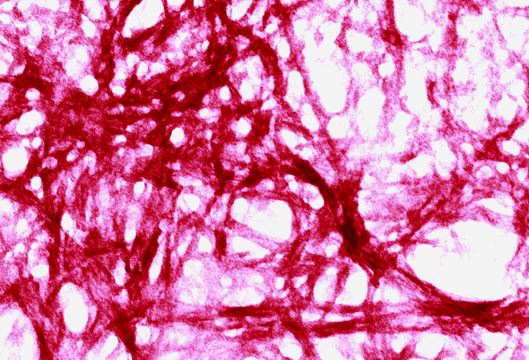 Fig 6.5 |
 Fig 6.2 |
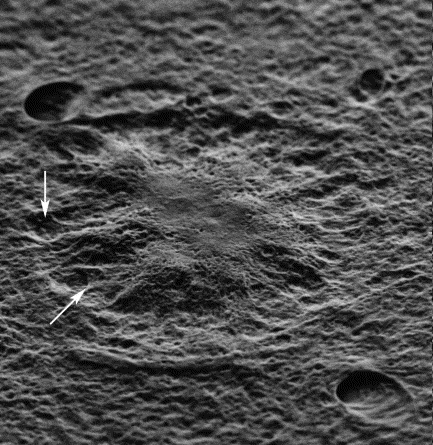 Fig 6.4 |
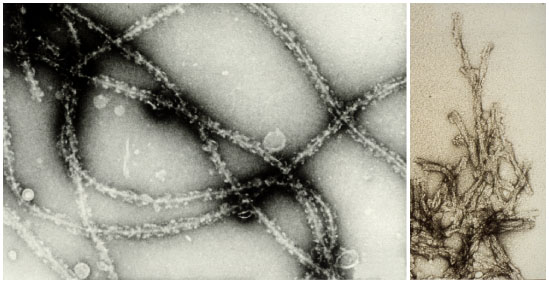 Fig 6.6 |
|
Fig 6.1 An electron micrograph of
deoxyHb S fibers formed in the presence of dextran (12 g/dl). |
||
|
fig. 6.3. (Upper) Electron micrograph of fibers
made of the glutamine- and asparagine-rich region of Sup35. images taken from: M. F. Perutz, |
||
|
Fig. 6.4 Scanning electron
micrograph of a classical amyloid plaque (AP) of a 23-month-old
Tg2576 mouse brain images taken from: G.Y. Wen, S.Y. Yang, W. Kaczmarski, X. Y. He and K. S. Pappas Presence of hydroxysteroid dehydrogenase type 10 in amyloid plaques (APs) of Hsiao's APP-Sw transgenic mouse brains, but absence in APs of Alzheimer's disease brains. Brain Res. 2002 Nov 1; 954(1):115-22. |
||
|
Fig 6.5 Unusual protein structures caused by: prion-like particles in yeast
|
||
|
Fig 6.6 Prion protein
fibers images taken from: Brown, D. R., Schmidt, B. and Kretzschmar, H. A (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380, 345-347 |
||
Our
approach
We primarily study sickle cell fiber
formation using spectroscopic methods.
X-ray crystallography has given us much information regarding the
atomic structure of sickle cell hemoglobin and provided important clues
regarding the association of individual molecules into fibers.
These data coupled with electron microscopy methods provide a
framework for the structure of the fibers.
Interestingly, in the crystals the fibers are linear, but in
solution the fibers exhibit a helical twist.
With the spectroscopic techniques that we are using in the
laboratory, we can now study the fibers as they form and elucidate
structural details in solution. We
have also been investigating different agents that inhibit fiber formation
and how it is done.
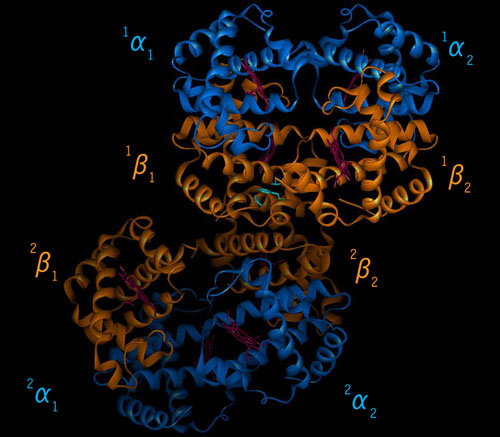
| Fig. 6.7 fibers of Hb S with all 8 sub-units. | |
|
|
|
(current research on Sickle Cell Disease)
(How do we study fiber formations?)