A molecule has 3N-6 vibrations
associated with it, where N is the number of atoms.
The 6 motions that are subtracted correspond to 3 translational and
3 rotational motions with zero frequency.
A linear molecule has 3N-5 vibrations, because one of the
rotational motions is lost due to the symmetry of the molecule.
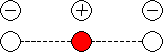
In the case of a non-linear triatomic molecule ABA, similar to H2O,
the three normal modes of vibration are:
symmetric stretching, symmetric bending and anti-symmetric bending (Fig
9.4) .
For a linear triatomic, 4 modes of vibration are predicted.
In contrast to the non-linear triatomic, a linear triatomic
molecule has an anti-symmetric stretching mode of vibration.
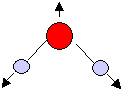 |
Symmetric stretching mode
|
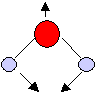 |
Symmetric bending mode
|
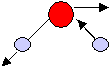 |
Antisymmetric bending mode |
| Fig 9.4 The 3 modes of vibration of a non-linear triatomic molecule ABA (i.e. H2O) | |
| Raman Spectrum of CO2 | |
| n1 | |
| n3 |
|
| n2a |
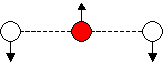 |
| n2b |
|
| FIG 9.5 The 4 modes of vibration of a linear triatomic molecule | |
Fig 9.11 |
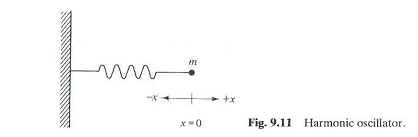 |
Each mode of vibration has a frequency
of oscillation associated with it. For
a diatomic molecule, a good analogy is a mass connected to a wall by a
spring (Fig
9.11). This
would be similar to a molecule like HCl or HBr, where the hydrogen atom is
much lighter than either Cl or Br. In
the absence of any forces, the mass on the spring is at its equilibrium
position – the length of the spring at rest.
If we stretch or compress the spring from its equilibrium position,
then classically we can talk about a restoring force associated with the
spring that will bring the mass back to its equilibrium position.
This strength of this restoring force will be dependent on the
relative stiffness of the spring, which can be described by a force
constant. In the case of a
bond, the force constant describes the relative strength of a bond so, for
example, a double bond will have a larger force constant than a single
bond. Classically, force, F=ma (mass ´
acceleration) and in the case of our spring,
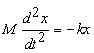 , where k is the force constant and x is the
displacement from the equilibrium position.
If we solve for x, then
, where k is the force constant and x is the
displacement from the equilibrium position.
If we solve for x, then
![]() . From this
relationship, we know that the frequency of a vibration is directly
related to the force constant of the bond and inversely related to the
masses of the interacting atoms. For
molecules bigger than diatomics the coupling of motions with other atoms
or vibrations also needs to be considered.
. From this
relationship, we know that the frequency of a vibration is directly
related to the force constant of the bond and inversely related to the
masses of the interacting atoms. For
molecules bigger than diatomics the coupling of motions with other atoms
or vibrations also needs to be considered.
(What is Raman Spectroscopy?)
(UVRR on HbA & HbS tetramers)