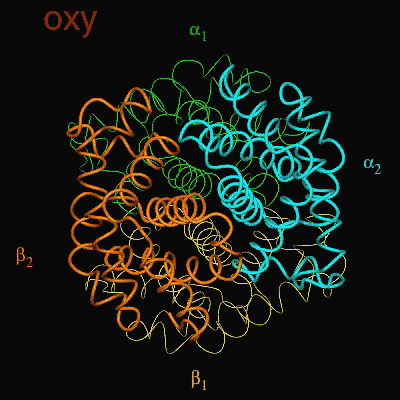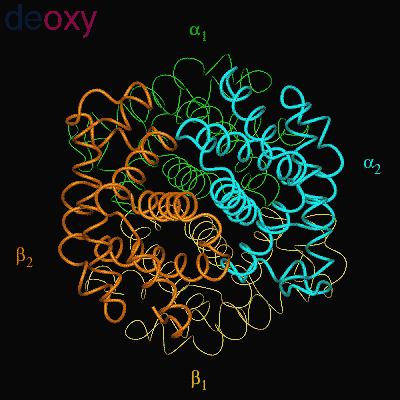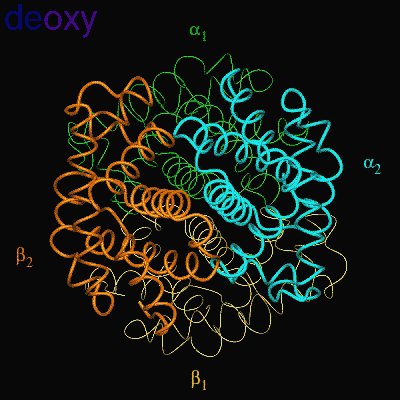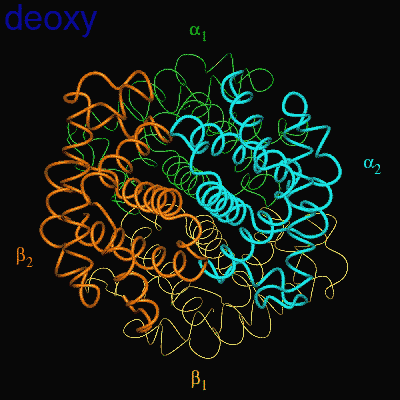|
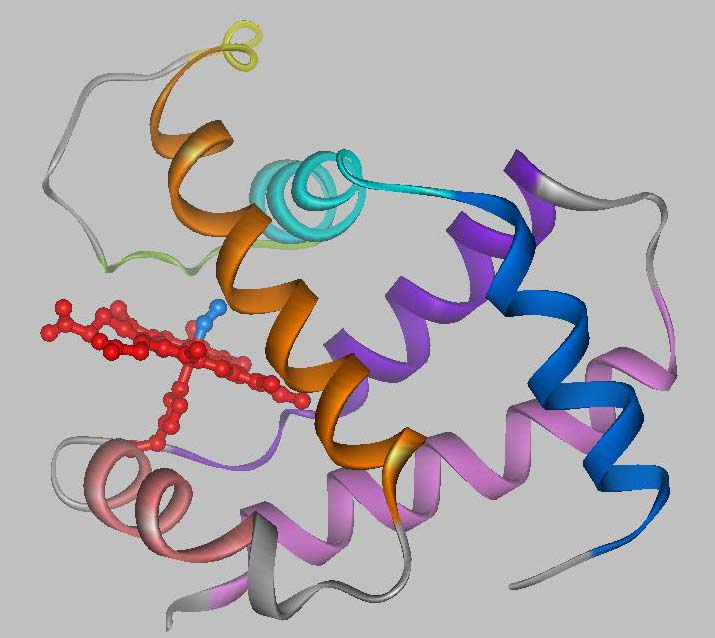
Hemoglobin is the oxygen carrier protein in red blood cells. It is also the protein, which gives red blood cells their red color. Hemoglobin consists of four subunits, two a and two b; each a and b subunit (refer to image) forms a dimer. Often, hemoglobin is referred to as a 'dimer of ab dimers.' The a and b subunits are only slightly different from each other with the main difference arising from the length of the polypeptide chains; the b chain is 5 amino acid residues longer than the a chain. There are also some differences in the composition of the amino acid residues. |
|
|
|
|
|
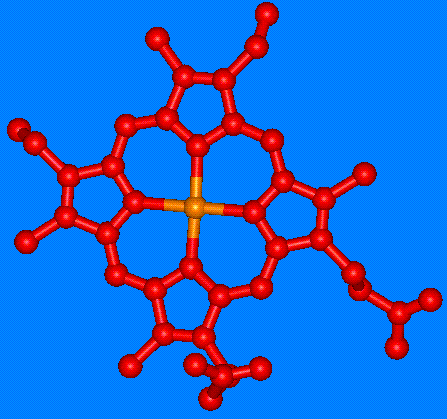
The figure at left shows the heme group, which contains an Iron (Fe) atom at the center (orange). In the oxy state, oxygen binds to the Fe in the heme group.
|
|
Each hemoglobin subunit contains a heme group. The heme group is the site of oxygen (O2) binding. When all four heme moieties bind O2, the structure of hemoglobin changes. This structural change involves a rearrangement of the ab dimers with respect to each other, where one ab dimer rotates approximately 18° and translates 1 Å with respect to the other dimer.
1Angstrom (Å) = 10-10m = 3.937 x 10-9 inches
|
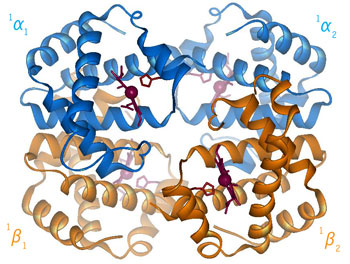
Fig 3.3 Graphic of T-R transition of HbS |
When the protein is in the structure that binds O2, the R-state, it binds O2 readily and when it is in the structure that has no O2 bound, the deoxy or T-state(Fig 3.3 and Fig 3.4), it doesn’t bind O2 very well. This difference in the ability to bind O2 depending on its structural state is what allows hemoglobin to be so efficient in delivering O2 to the tissues. Once it delivers O2, the structural state changes and it will not bind the delivered O2. When the Red Blood Cells (RBCs) return to the lungs, the O2 concentration is higher and hemoglobin binds O2 again and changes its structural state. Understanding how this structural change is effected is a central question in current hemoglobin research.
|
|
|
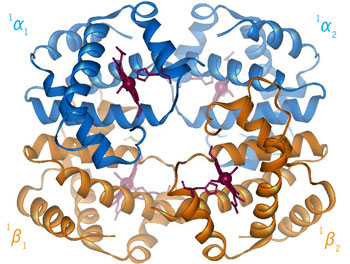
Fig 3.4
Relative Motion of Dimers image from Jonathan Lukin, Dept. of Biological Sciences, Carnegie Mellon University http://www.andrew.cmu.edu/user/jl2p/Hb_html/gallery.html |