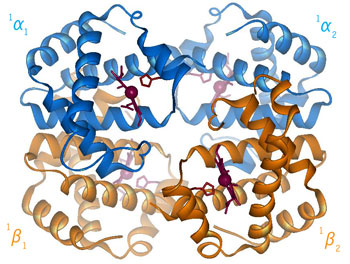
In sickle cell hemoglobin, fiber formation occurs when individual hemoglobin molecules stick together. This process happens in sickle cell hemoglobin because of the genetic mutation that leads to a change in a single amino acid residue of the protein sequence. In sickle cell hemoglobin the changed or mutated amino acid is hydrophobic. The literal meaning of hydrophobic is 'fear of water.' This is not exactly the case in hemoglobin.
This amino acid change occurs on the surface of the sickle cell hemoglobin Since the cellular environment of red blood cells is similar to water, the amino acid change causes the hemoglobin to associate with other hemoglobin molecules rather than with the cellular environment. This preferential interaction of the hemoglobin molecules with each other rather than water is attributed to a 'hydrophobic interaction' of certain amino acid residues, one of which is the mutated residue, b6 Valine.
|
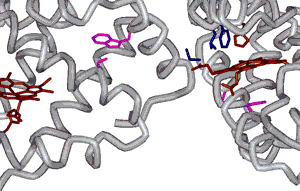
This figure (left) depicts the interaction between hemoglobin molecules that is thought to drive fiber formation. The amino acid residues that interact with each other, b6 Valine, b85 Phenylalanine and b88 Leucine, are shown in blue. The b6 Valine comes from one b subunit from one hemoglobin while the b85 Phenylalanine and b88 Leucine residues come from a b subunit of a different hemoglobin molecule. Also shown are heme groups with coordinating His residues (maroon). |
Going deeper: hydrophobic interactions of amino acids
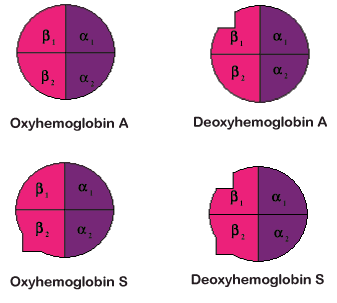
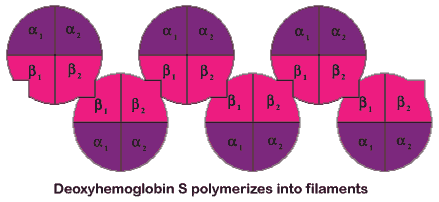
| Fiber formation only occurs in the deoxy or T-state. Because of the structural change to form the T-state, a different region of the protein exposes a hydrophobic surface area. The area containing the mutated amino acid residue and the area exposed by forming the T-state associate together to form the fibers. Hemoglobin molecules associate into double strands and then, seven of these strands associate together to form a fiber. |
Hemoglobin (HbS) in T- State |
||||
|
|
|||||
 |
These fibers then associate with
each other forming larger and larger fibers over time.
These fibers can be observed using a technique called
electron
microscopy. The double
strands of sickle cell hemoglobin fibers have been observed using
X-ray
crystallography. (Fig 4.5) |
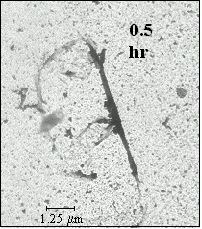
| FIG 4.5 electron micrograph of fiber with heterogeneous nucleation |
Fiber formation occurs through two main stages, referred to as the double nucleation mechanism (Fig 4.6). In the first stage individual hemoglobin molecules have to come together to form a nucleus, that will be the initiation point for the fibers. Approximately, seven hemoglobin molecules need to come together to form this nucleus. This process is relatively slow, because of the number of molecules that need to come together to form the nucleus. Once the nucleus is formed the second stage of the process begins. In this second stage, fiber formation is relatively rapid because fibers can build on the pre-existing structure. Practically, this means that it is not necessary to bring so many molecules together at once, which significantly speeds up the process.
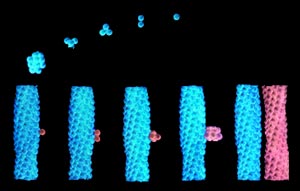 FIG 4.6 Double nucleation mechanism graphic shows the two stages of fiber formation upper half -1st stage; lower half - 2nd stage image taken from http://k12education.uams.edu |
Our
laboratory is interested in understanding the mechanics of the first or
initial stage of the fiber formation process.
If this process can be extended to match that of the circulation
time in the body, then the Red Blood Cells (RBCs) would become exposed to oxygen before a
significant amount of fibers could form.
We believe that this would be extremely beneficial in the treatment
of sickle cell disease. (link to the research pages)